Lipid Nanoparticles (LNPs) are the most advanced nucleic acid delivery system currently available. Having experienced a massive surge in popularity due to their use in mRNA vaccines during the COVID-19 pandemic, LNPs are at the forefront of scientific research and a wildly hot topic among the scientific community. Now you’re asking how LNPs can enhance your research and, with LipExoGen, the possibilities are endless. Our LNP Synthesis services allow you to encapsulate and deliver mRNA, siRNA, ASOs or other cargos to specific cells or tissues. Our experts know how to tune the LNP properties to achieve long-circulation, accumulation in tumor tissues, and preferential uptake in target cells or tissues. We provide our clients unparalleled customization to surface modify the LNPs with antibodies, proteins, peptides, aptamers, and more. Fluorescent labels can be integrated to facilitate in vivo biodistribution studies or cellular uptake assays. Our passion for basic research and strong ability to think outside the box is why our clients say working with us feels like a true collaboration. Whether you’re developing next-generation vaccines, mRNA LNPs, or something totally new, we have the expertise to drive your success. Contact us today and see first-hand why we’re the best.
For research use only
Target Any Gene, Target Any Cell
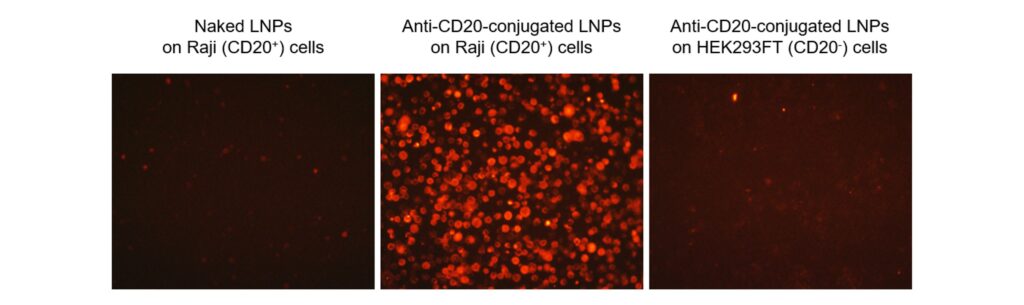
Figure 1. Specific uptake of LNPs in target cells. LNPs were synthesized containing 0.1% of the fluorescent dye DiI (red) and further modified to reduce non-specific cellular uptake. Last, LNPs were either surface modified by an anti-human CD20 antibody or left unmodified (naked) and applied to cells for 24 h before acquiring fluorescence microscopy images. Naked LNPs are not efficiently taken up by Raji cells because the LNPs were engineered to reduce non-specific cellular uptake (left). Conjugating an anti-CD20 antibody to the surface of the LNPs promotes their specific uptake in Raji cells, which are CD20+ (middle), but not in HEK293FT cells, which are CD20- (right). Our formulation experts can encapsulate the nucleic acid cargo of your choosing and surface modify the LNPs with any antibody you want to produce similar effects depending on the cells you wish to target.
 Figure 2. Schematic components of mRNA LNPs. Lipid nanoparticles generally contain four major types of lipids. An ionizable lipid, such as MC3, constitutes the bulk of the lipid components and is responsible for the encapsulation of mRNA or other nucleic acid cargo. Under LNP synthesis conditions, the ionizable lipid is protonated and bears a positive charge which interacts with the negative charge of nucleic acids. At physiological pH, it is deprotonated and neutral, thus overcoming the drawback of cationic liposomes which are inherently toxic. Once endocytosed, the ionizable lipid becomes positively charged again and promotes membrane fusion allowing the cargo to be ejected into the cytosol. Helper lipids, such as DSPC and DOPE, play structural roles and are involved in the process of endosomal escape. Cholesterol is typically the second most abundant lipid component, which plays a structural role. A PEGylated lipid such as DMG-PEG2000 stabilizes the LNPs and allows small particle sizes to be achieved. Stability is achieved by the steric effect afforded by polyethylene glycol (PEG) polymer which is covalently linked to the lipid headgroup. The amount and type of PEG lipid is important for determining the cellular fate of the LNPs and their in vivo biodistribution.
Figure 2. Schematic components of mRNA LNPs. Lipid nanoparticles generally contain four major types of lipids. An ionizable lipid, such as MC3, constitutes the bulk of the lipid components and is responsible for the encapsulation of mRNA or other nucleic acid cargo. Under LNP synthesis conditions, the ionizable lipid is protonated and bears a positive charge which interacts with the negative charge of nucleic acids. At physiological pH, it is deprotonated and neutral, thus overcoming the drawback of cationic liposomes which are inherently toxic. Once endocytosed, the ionizable lipid becomes positively charged again and promotes membrane fusion allowing the cargo to be ejected into the cytosol. Helper lipids, such as DSPC and DOPE, play structural roles and are involved in the process of endosomal escape. Cholesterol is typically the second most abundant lipid component, which plays a structural role. A PEGylated lipid such as DMG-PEG2000 stabilizes the LNPs and allows small particle sizes to be achieved. Stability is achieved by the steric effect afforded by polyethylene glycol (PEG) polymer which is covalently linked to the lipid headgroup. The amount and type of PEG lipid is important for determining the cellular fate of the LNPs and their in vivo biodistribution.
What Are LNPs
LNPs stands for Lipid Nanoparticles. They are a type of delivery system used in pharmaceutical and biotechnology industries to encapsulate and deliver various therapeutic molecules, particularly nucleic acids including messenger RNA (mRNA), small interfering RNA (siRNA), microRNA (miRNA), antisense oligonucleotides (ASOs), single guide RNA (sgRNA), DNA, and others.
LNPs are composed of lipids, which are natural or synthetic molecules that have both hydrophilic (water-attracting) and hydrophobic (water-repellent) properties. These lipids can self-assemble into nanoparticles when mixed with the therapeutic molecules. The lipid nanoparticles protect the therapeutic cargo and enhance its stability, allowing it to be efficiently delivered to target cells or tissues.
LNPs typically contain an ionizable lipid that serves to encapsulate the nucleic acids, along with helper lipids and cholesterol which play structural roles and facilitate endosomal escape, and a PEGylated lipid which provides stability to the LNPs.
To produce LNPs, two solutions (one containing lipids and the other containing the cargo) are directed to specific channels or chambers where they are brought into contact and mixed. The channels may have complex geometries or feature structures like obstacles or grooves to enhance the mixing efficiency.
As the lipid and cargo solutions mix, the organic solvent in the lipid solution rapidly diffuses into the aqueous phase. This process leads to the formation of LNPs, where the lipids self-assemble around the cargo, encapsulating it within lipid bilayers or vesicles. The resulting LNPs are collected from the microfluidic chip and further processed for purification. Purification methods, such as tangential flow filtration, are then employed to remove any remaining impurities, unencapsulated cargo, and solvent, and to adjust the buffer solution and mature the LNPs.
Downstream processing such as PEGylation or conjugation of targeting ligands including peptides, aptamers or monoclonal antibodies allow the resulting LNPs to be fine-tuned for the desired properties.

Figure 3. Schematic representation of the process for LNP synthesis. Nucleic acid cargo and lipids are first dissolved at an appropriate concentration in separate solutions. The controlled mixing of the aqueous and organic phases results in lipid nanoparticle self-assembly and encapsulation of the therapeutic molecule such as mRNA. An ionizable lipid bears a positive charge under specific pH values used for lipid nanoparticle synthesis, but is deprotonated at physiological pH making it neutral. During LNP synthesis, the charge interaction between ionizable lipid and the phosphates present in nucleic acids drives encapsulation of the nucleic acid. Following LNP synthesis, buffer exchange is carried out to adjust the ionic strength and pH of the bulk solution for physiological applications. Surface modification with monoclonal antibodies or other proteins, peptides, aptamers, or other functional molecules can be performed to fine-tune the LNP properties. Fluorescent dyes can be included in the formulations to allow study of cellular uptake or in vivo biodistribution using IVIS, flow cytometry, fluorescence microscopy, and other common laboratory techniques (not shown).
Why LNPs
- LNPs are the most clinically-advanced nucleic acid delivery vector
- Enhanced cellular uptake: LNPs (Lipid Nanoparticles) offer improved cellular internalization and delivery of therapeutic cargo, enabling efficient delivery of nucleic acids, proteins, and small molecules to target cells.
- Versatile cargo delivery: LNPs can efficiently encapsulate a wide range of cargo, including mRNA, siRNA, DNA, and small molecules, allowing for versatile applications in drug delivery.
- Stability and protection: LNPs provide stability and protection to the cargo during transit, shielding it from degradation by enzymes and immune responses, thereby enhancing the therapeutic efficacy.
- Targeted delivery: LNPs can be engineered to incorporate targeting ligands, such as antibodies or peptides, facilitating specific binding to target cells or tissues, thereby increasing the efficiency of delivery and reducing off-target effects.
- Scalability: LNPs can be manufactured at large scales using reproducible and scalable methods, making them suitable for both research and potential clinical translation.
- Biocompatibility and safety: LNPs are generally considered biocompatible and safe for use in research and potentially in clinical applications, as they can be engineered to minimize toxicity and immunogenicity.
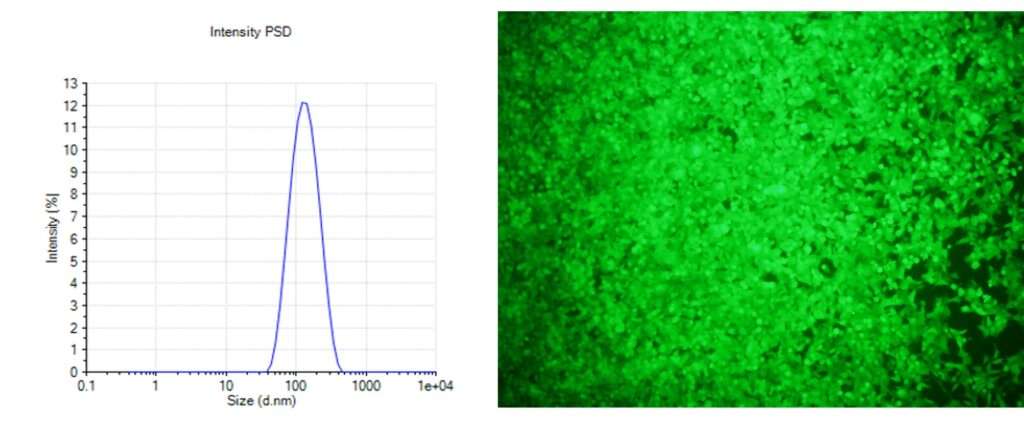
Figure 4. EGFP mRNA LNPs in action. In vitro transfection of HEK293FT cells with EGFP mRNA LNPs is shown by fluorescence microscopy. The size distribution on the left shows the average particle diameter determined by dynamic light scattering (DLS). Note: in this example the LNPs were not engineered to reduce non-specific cellular uptake – therefore, no antibody or targeting molecule is required.
- Tissue penetration: LNPs can be engineered to traverse various biological barriers, including the blood-brain barrier, improving the delivery of therapeutics to target tissues that were previously difficult to reach.
- Enhanced stability and half-life: LNPs can increase the stability and half-life of cargo molecules in the bloodstream, prolonging their availability for therapeutic action.
- Modifiability: LNPs can be easily modified and optimized for specific cargo and target cells, enabling researchers to tailor their properties for enhanced delivery efficiency and therapeutic efficacy.
Why Choose Us
Harnessing the Power of mRNA LNPs for Transformative Therapeutics: At LipExoGen, we are at the forefront of mRNA LNP synthesis, providing a comprehensive range of services to unlock the full potential of mRNA-based therapeutics. Our team of skilled scientists and engineers specializes in developing customized LNP formulations that enable precise and efficient delivery of mRNA to target cells. Whether you’re a researcher, a pharmaceutical company, or a government agency, our services offer you the tools to accelerate your scientific discoveries and translational applications.
Tailored Solutions for Your Research and Development Needs: With our mRNA LNP synthesis services, we empower researchers across academia, industry, and government to explore new frontiers in molecular medicine. We understand that each project is unique, requiring specific characteristics and performance criteria. Our experienced team collaborates closely with clients to design and synthesize LNPs customized to their precise specifications, ensuring optimal encapsulation, stability, and delivery of mRNA payloads. Whether you’re developing next-generation vaccines, RNA-based therapeutics, or something else, we have the expertise to drive your success.
Cutting-Edge Technology and Unparalleled Expertise: At LipExoGen, we combine state-of-the-art technology with deep scientific knowledge to deliver outstanding results. Our proprietary mRNA LNP synthesis platform employs innovative lipid formulations and scalable manufacturing processes, enabling us to produce high-quality LNPs with excellent reproducibility. We leverage our expertise in materials science, nanotechnology, and mRNA biology to optimize LNP characteristics such as size, charge, and surface modifications, tailoring them to specific applications and overcoming delivery challenges.
Accelerating Drug Development and Translation: We recognize the urgency of translating groundbreaking research into clinical applications. With our mRNA LNP synthesis services, we help expedite the drug development process, bridging the gap between discovery and clinical trials. Our streamlined workflows and rigorous quality control measures ensure that you receive reliable, reproducible LNPs for preclinical studies, enabling you to achieve critical milestones and advance your therapeutic candidates efficiently.
- Partnering for Success: Collaboration is at the heart of our approach. We foster strong partnerships with our clients, working closely with you to understand your unique requirements, timelines, and budget constraints. Our dedicated team provides expert guidance throughout the project, from initial design to synthesis, characterization, and beyond. Together, we can overcome challenges, innovate new solutions, and accelerate the development of transformative mRNA-based therapies that can revolutionize patient care and public health.
Our Capabilities
Encapsulate a wide variety of cargo:
- Messenger RNA (mRNA)
- Small interfering RNA (siRNA)
- Single guide RNA (sgRNA)
- Micro RNA (miRNA)
- Antisense oligonucleotide (ASO)
- ODN CpG
- DNA
Surface modify for specific targeting and other properties:
- Monoclonal antibodies
- scFV or Fab
- Nanobodies
- Proteins
- Cytokines
- Protein functional domains
- Fusion proteins
- Peptides (e.g. cRGD)
- Aptamers
- Glycosylation
- Small molecule ligands (e.g. folate, etc.)
- Reduction of non-specific uptake
- Incorporate fluorescent dyes for in vivo imaging and cellular uptake assays
Strong manufacturing capabilities
- Microfluidics for scalable control over particle size and PDI
- Microliter to liter scales
- Dynamic light scattering (DLS) analysis of particle size (Z-average) and polydispersity index (PDI)
- Zeta potential, conductivity
- Concentration
- Encapsulation efficiency (EE)
- HPLC, Ribogreen Assay
- In vitro Functional Assays (transfection efficiency, cytotoxicity assays, etc.)
- SDS-PAGE with Coomassie blue or Western blot for validation of antibody conjugation
- Stability assays
- Sterile filtration with 0.22 micron PES
- Dispensed in sterile glass vials free of endotoxin, pyrogens, DNAse and RNAse, stoppered and crimp-capped under sterile conditions
Additional Services
- Low-risk and low cost pilot (feasibility) studies
- Preliminary data packages for grant applications (academic only)
- Site-specific antibody conjugation (linker restricted to Fc-region)
Get Started...
Email: info@lipexogen.com
Call: 410-231-3496
All products and services are for preclinical research only, not intended for human consumption, veterinary or diagnostic purposes.
