-
Previous

Human PDGFD shRNA Lentivirus
$595.00 – $1,195.00
-
Next

YAP1 cDNA Plasmids
$595.00
Fluorescence and Luciferase Dual Reporter Lentivirus
$595.00
Fluorescence and luciferase dual imaging reporter lentivirus: Collection of high-quality reporter lentiviral particles which allow for detection of transduced cells by luciferase and fluorescence based methods. The vector constructs contain self-cleaving peptides for independent protein expression of the luminescent (firefly luciferase or secreted Gaussia luciferase) and fluorescent (GFP or RFP) reporters from a single mRNA transcript. The lentiviral particles are ultra-purified and concentrated to high-titer by PEG precipitation and sucrose gradient centrifugation, and are ideal for transduction of difficult-to-transfect cells including primary and/or thawed cells. These versatile lentiviral reporters provide an incredibly powerful tool for studying tumor growth and metastasis in preclinical models of cancer.
Key Advantages:
- Dual-Readout – Get the most data from your experiments by exploiting luciferase and fluorescent reporters in the same cells. Easily transduce primary tumor cells or tumor cell lines and use luciferase imaging to track tumor growth and metastasis in vivo. Then, use the GFP or RFP reporter to identify the tumor cells on flow cytometry from dissociated tumors. The fluorescent reporters are excellent for gating out tumor cells for cleaner analysis of tumor-infiltrated lymphocytes (TILs), tumor associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), and other infiltrated immune subsets. Alternatively, they can be used to sort or exclude tumor cells by FACS prior to scRNA-seq, proteomics analysis, and other downstream applications. Fluorescence microscopy can also be used to monitor living cells.
- Optimized Vectors – LipExoGen lentiviral particles are made using a novel vector platform based on the third generation system. Expression of the luminescent and fluorescent reporters and drug selection markers is driven by CMV, PGK, or EF1a promoter, and the genes are multicistronically expressed as individual proteins mediated by self-cleaving peptides. Multiple vector layouts are available, and different combinations of promoter, reporter, and drug selection are summarized in the table below.
- Ultra-Purified & High-Titer – Dual-imaging lentiviral particles are ultra-purified and concentrated to high-titer by PEG purification and sucrose gradient centrifugation, and are suitable for both in vitro and in vivo applications.
- Easily Transduce Primary Cells – Dual-imaging lentiviral particles can efficiently transduce primary tumor cells as well as bone marrow stem cells, lymphocytes, monocytes, neurons, and other difficult-to-transfect cells. The particles are also effective for transduction of freeze-thawed cells.
- Easily Establish Stable Cell Lines – Stable cell lines are easily generated through puromycin or blasticidin selection. Alternatively, transduced cells can be FACS sorted based on expression of GFP or RFP.
Multiple vector layouts and combinations of promoter/reporter/drug selection are available, summarized below. Products with vector layouts LTV-V1 and LTV-V2 are the most effective for establishing stable cell lines. For applications where transduction of primary cells followed by FACS sorting is the primary focus, products with vector layouts LTV-V3 and LTV-V4 are recommended. To view the different vector layouts, click here.
Available Options:
Specifications
Background
Luciferase is widely used as a reporter for both in vitro and in vivo studies in the life sciences. For in vivo preclinical studies, it is a gold-standard for monitoring the growth and spread of tumors which are not readily detectable by calipers or other form of direct observation (1). It is particularly useful for observing tumor progression in orthotopic mouse models of hematological malignancies and brain tumors, which would be introduced by intravenous and stereotactic injection, respectively. Orthotopic models of metastasis can also be visualized for certain cancers following removal of the primary tumor. In vitro, fluorescence is more suitable as a reporter because it can be easily analyzed at single cell resolution using flow cytometry and allows for FACS sorting.
LipExoGen Luciferase/Fluorescent Dual-Imaging Lentiviruses use a single, efficient construct containing both luciferase and a fluorescent reporter (GFP or RFP) to enable the detection of bioluminescence and fluorescence in the same cells. The high-titer lentiviral particles are ultra-purified and transduce human or mouse cells with high efficiency, which can be easily monitored by fluorescence microscopy (Fig. 1). After transduction, FACS sorting or drug selection (puromycin or blasticidin) makes it easy to establish stable cell lines. Tumor growth is easily monitored in preclinical animal models involving intravenous, stereotactic, or local injection of tumor cells (Fig. 2). Metastasis can also be studied in vivo or ex vivo (Fig. 3). Once the tumor cells have been harvested, they can be subsequently be analyzed by detecting GFP or RFP on flow cytometry in combination with immunoprofiling by antibodies specific for tumor-infiltrated immune cells. Pure tumor cells from immune-competent animals treated with different agents can be rapidly isolated by FACS to perform downstream analyses such as scRNA-seq, RT-PCR, or proteomics, without contamination of infiltrated immune cells or fibroblasts. The lentiviral particles can be combined with compatible Transcription Factor (TF) Reporter Lentiviruses to study signaling pathway activation in the tumor cells after isolation from in vivo models. Collectively, these features make Luciferase/Fluorescent Dual-Imaging Lentiviruses an incredibly powerful and versatile tool to boost your preclinical studies and pave the way for discovery.
Product Data
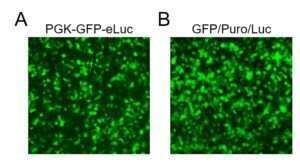
Figure 1. Transduction efficiency HEK293FT cells (1-3×10^5, 24-well) were transduced with the indicated lentivirus (20 µL) for 36 hours prior to image acquisition by fluorescence microscopy for visualization of transduction efficiency via the GFP reporter.
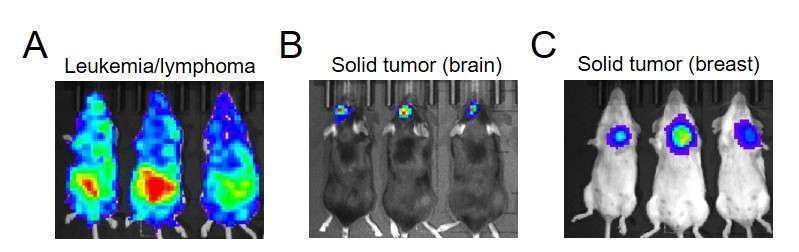
Figure 2. In vivo luciferase imaging of primary tumors Tumor cells (1-3×10^5, 24-well) were transduced with 20 µL of lentivirus for 36 hrs, then sorted for GFP or RFP (depending on vector used) by FACS. After expanding in vitro, the sorted cells were delivered to recipient mice. After 10-20 days of engraftment, bioluminescence imaging was performed using IVIS 10 minutes after intraperitoneal injection of D-luciferin (50 mg/kg). A. Luciferase imaging of hematological tumors. TIB-23 myeloma cells were imaged in BALB/c recipients on day 20 after tumor cell inoculation (1×10^6 cells/mouse, i.v.). SKU LFV-0001-11. B. Luciferase imaging of brain tumors. GL261 glioma cells were imaged in C57BL/6 recipients 10 days after stereotactic injection of tumor cells (1×10^5/mouse). SKU LFV-0001-4. C. Luciferase imaging of solid tumors. 4T1 triple-negative breast cancer cells were imaged in BALB/c recipients 20 days after orthotopic (mammary fat pad) injection of tumor cells (1×10^6/mouse). SKU LFV-0001-13.
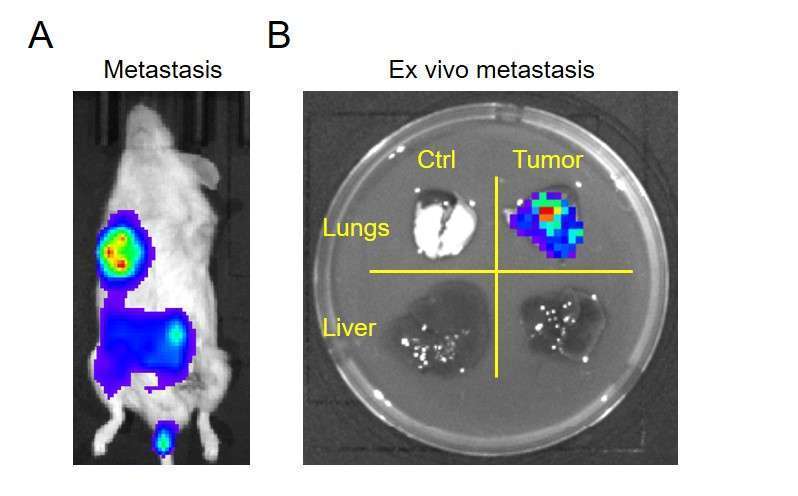
Figure 3. In vivo and ex vivo luciferase imaging of tumor metastasis SUM159 human breast cancer cells (1-3×10^5, 24-well) were transduced with 20 µL of lentivirus (SKU LFV-0001-1) for 36 hrs and selected by puromycin. NSG mice received 1×10^6 tumor cells by orthotopic injection to the lower mammary fat pad. Ten days later, 5×5 mm tumors were excised. A. Metastasis, visualized in vivo 14 days after removal of the primary tumor by luciferase imaging (D-luciferin, 50 mg/kg). B. Ex vivo luciferase imaging of lung and liver tissues, revealing tumor metastasis in lung but not liver (right panels). Left panels are from a control mouse (no tumor) which also received D-luciferin injection.

Figure 4. In vivo transduction of skin cells with ultra-high titer lentiviral particles A nude mouse received 10 uL of ultra-high titer Fluorescence and Luciferase Dual Reporter Lentivirus (SKU LFV-0001-6) subcutaneously and in vivo imaging with luciferin was performed a few days later. Ultra-high titer concentration is available upon request (contact us).

Figure 5. CMV-RFP-BSD-Luc representative image Customer deposited imaging data showing the luminescence signal in NSG mice that received stable MOLM-14 cells created with CMV-RFP-BSD-Luc (SKU LFV-0001-08).

Figure 6. Detection of Gaussia Luciferase in the Serum MOLM13 cells were transduced with PGK-GFP-Luc (LTV-0001-11) and selected by FACS for GFP. Next, the cells were transduced with CMV-GLuc-RFP-Puro and selected with puromycin to establish the stable cell line expressing both Firefly and Gaussia Luciferase. The FLuc/GLuc dual reporter MOLM13 cells were injected i.v. into NSG mice and Firefly luciferase was imaged by the IVIS 5 and 13 days later (A). On the same days, serum was collected from the peripheral blood and analyzed for Gaussia Luciferase activity using a commercially available Gaussia Luciferase Assay Kit and luminometer (5 μl of serum per test). The data is meant to show that GLuc can be detected in serum for convenient measurement of cell growth, particularly for investigators who do not have access to specialized IVIS equipment. Because GLuc is secreted, its serum activity can be assayed to monitor the growth of either liquid or solid tumors. This same principle could be applied for our other Signaling Pathway & TF Reporter Products, and Immunotherapy Reporter Products, which are also available with GLuc Readouts.
Guide in selecting imaging reporter variants
Different products in our Fluorescence and Luciferase Dual Reporter Lentivirus product collection, which feature different combinations and arrangements of fluorescent and luminescent reporters, drug selection markers, and promoters, have been tested for their relative levels of expression and lentivirus titers. The following guidelines are provided to help you in choosing which product variant is best suited to your particular application.
Among different promoters (CMV, PGK, or EF1a), CMV drives much stronger expression of each component so placing luciferase in the last is to avoid the possible influence of P2A or T2A to luciferase performance in vivo. As a result, the luciferase in LFV-V1b vector works enough for in vivo imaging while maintaining the first-place fluorescent protein brighter for fluorescence imaging. EF1a promoter drives weaker expression of each component than CMV, thus likely avoiding the influence of fluorescence and luminescence on cell performance such as stem cells and lymphocytes. This might be the reason to choose EF1a promoter for these cells rather than CMV promoter. PGK promoter performs in the middle between the CMV and EF1a so that it might be universal. Fluorescent protein expression is better in the combination of two components than three components so that for difficult transducing primary and stem cells, which cannot be stood up with drug selection, the EF1a or PGK promoter driving fluorescence and luminescence without drug is the better choice for flow-sorting transduced cells. The transducing efficiency of blasticidin is better than puromycin in most types of cells and the cytotoxicity of blasticidin is weaker than puromycin, so that low dose puromycin is recommended to be used at first for selection after transduction, then increasing puromycin dose for strong expression of fluorescence and luminescence. Therefore, blasticidin drug might be better for primary cells than puromycin.
Custom Orders
If you require a modification to one of our products (for example, change in reporter or other vector component), please request a custom order. We provide a variety of fast and efficient services for the production of high-quality, custom lentiviral particles on demand, usually for the same or comparable price as the listed item.
I want to:
View vector layouts for luciferase/fluorescent dual-reporter lentiviruses